how to distinguish between aldehyde and ketone Ketones & aldehydes test
Starting my day with some exciting information on organic chemistry! Today, I stumbled upon some fascinating information regarding the carbonyl functional group that is present in aldehydes and ketones. To put it simply, the carbonyl group consists of a carbon atom and an oxygen atom that are double-bonded together. This functional group is the reason why aldehydes and ketones have such unique chemical properties. Let’s start with aldehydes. These compounds are characterized by the presence of a carbonyl group at the end of a carbon chain. The simplest aldehyde is known as formaldehyde, which is commonly used in the production of plastics and resins. When naming aldehydes, they are typically named by replacing the “e” at the end of the parent alkane with “-al”. For example, the aldehyde with two carbons is known as acetaldehyde. Now let’s move on to ketones. These compounds are characterized by the presence of a carbonyl group in the middle of a carbon chain. A simple example of a ketone is known as acetone, which is used as a solvent in many industries. When naming ketones, they are typically named by replacing the “e” at the end of the parent alkane with “-one”. For example, the ketone with three carbons is known as propanone (also called acetone). But why are aldehydes and ketones so interesting? Well, it turns out that the carbonyl group is highly reactive and undergoes a variety of chemical reactions. Aldehydes and ketones can react with a wide range of compounds, such as alcohols, amines, and carboxylic acids. These reactions can lead to the formation of new functional groups and the creation of more complex organic molecules. To better understand these reactions, let’s take a look at a common test used to distinguish between aldehydes and ketones. This test is known as the “Tollens’ test” and involves the oxidation of an aldehyde in the presence of a silver catalyst. The resulting product is a “silver mirror” that forms on the inside of the test tube. Ketones, on the other hand, do not react in this test and do not produce a silver mirror. Overall, aldehydes and ketones are fascinating compounds that have a wide range of uses and chemical properties. They play important roles in the production of many everyday products, such as plastics, solvents, and pharmaceuticals. By understanding the chemistry behind these compounds, we can better appreciate the complexity and beauty of organic chemistry. And that’s a wrap! I hope you enjoyed learning about aldehydes and ketones as much as I did. Until next time!
Image 1: Aldehydes and Ketones
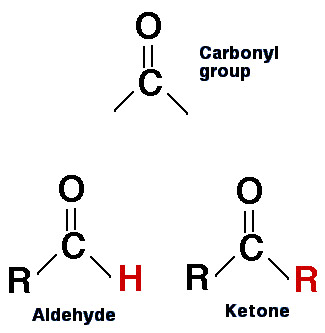 Image Source: https://biology.reachingfordreams.com/images/Chemistry/Organic_chemistry/Aldehydes_Ketones/aldehyde_and_ketone.jpg
Image Source: https://biology.reachingfordreams.com/images/Chemistry/Organic_chemistry/Aldehydes_Ketones/aldehyde_and_ketone.jpg
Image 2: Ketones & Aldehydes Test

If you are searching about Aldehydes and Ketones: the carbonyl functional group, naming, reactions you’ve came to the right web. We have 5 Pics about Aldehydes and Ketones: the carbonyl functional group, naming, reactions like How will you distinguish between aldehyde and ketone?, Difference Between Aldehyde and Ketone | Structure, Properties, Naming and also Difference Between Aldehyde and Ketone | Structure, Properties, Naming. Here you go:
Aldehydes And Ketones: The Carbonyl Functional Group, Naming, Reactions
 biology.reachingfordreams.comketone ketones aldehydes carbonyl group aldehyde functional structure difference between carboxylic compound molecule acids biology groups chemistry two reactions diagram
biology.reachingfordreams.comketone ketones aldehydes carbonyl group aldehyde functional structure difference between carboxylic compound molecule acids biology groups chemistry two reactions diagram
Pin By Flow - Wellness And Training On Quick Saves In 2021 | Organic
 www.pinterest.com.mxHow Will You Distinguish Between Aldehyde And Ketone?
www.pinterest.com.mxHow Will You Distinguish Between Aldehyde And Ketone?
 chemistrypage.inaldehyde ketone distinguish chemistrypage
chemistrypage.inaldehyde ketone distinguish chemistrypage
Ketones & Aldehydes Test

Difference Between Aldehyde And Ketone | Structure, Properties, Naming
 in.pinterest.comaldehyde ketone ketones pediaa aldehydes keton aldehyd carboxylic unterschied
in.pinterest.comaldehyde ketone ketones pediaa aldehydes keton aldehyd carboxylic unterschied
Ketone ketones aldehydes carbonyl group aldehyde functional structure difference between carboxylic compound molecule acids biology groups chemistry two reactions diagram. How will you distinguish between aldehyde and ketone?. Pin by flow